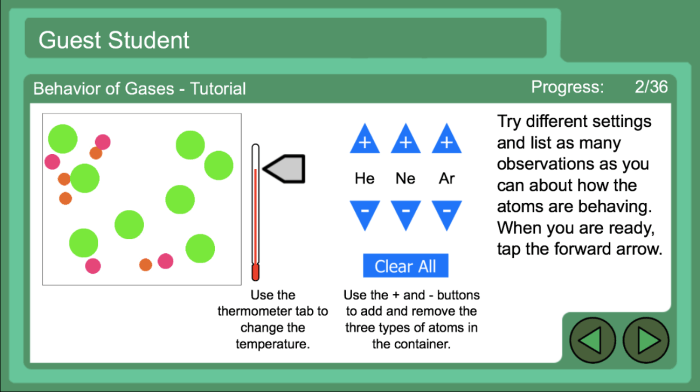
Behavior of gases worksheet answer key: Embark on a scientific voyage into the realm of gases, where we unravel the mysteries of their behavior and uncover the fundamental principles that govern their interactions. Prepare to witness the interplay of kinetic molecular theory, gas laws, and real gas deviations, all meticulously explained with captivating examples and thought-provoking insights.
From the everyday phenomena we encounter to the intricate workings of the universe, gases play a pivotal role in shaping our world. This worksheet delves into the very essence of gas behavior, empowering you with a comprehensive understanding of these elusive substances.
Behavior of Gases
Gas behavior is a fundamental aspect of physical chemistry that describes the physical properties and interactions of gases. Gases exhibit unique characteristics that differentiate them from solids and liquids, and understanding their behavior is crucial in various scientific and engineering applications.
Gas behavior can be observed in everyday life through phenomena such as the expansion of air in a balloon when heated, the pressure buildup in a sealed container of gas, and the diffusion of gases through porous materials. These observations provide insights into the fundamental principles governing gas behavior.
Factors Influencing Gas Behavior
- Temperature: Temperature significantly affects the kinetic energy and molecular motion of gases, influencing their volume, pressure, and other properties.
- Pressure: Pressure exerts an external force on gases, affecting their volume and density. Increasing pressure compresses gases, while decreasing pressure allows them to expand.
- Volume: The volume of a gas refers to the space it occupies. Changes in volume are directly related to changes in pressure and temperature.
Kinetic Molecular Theory

The kinetic molecular theory (KMT) is a fundamental theory that explains the behavior of gases by describing their molecular composition and motion. According to the KMT, gases consist of tiny, spherical molecules that are in constant random motion.
The KMT explains gas behavior by considering the following postulates:
- Gas molecules are in constant random motion, colliding with each other and the walls of their container.
- The average kinetic energy of gas molecules is directly proportional to the absolute temperature of the gas.
- Gas molecules are assumed to be point masses with negligible volume.
The KMT provides a framework for understanding gas behavior, including phenomena such as diffusion, effusion, and the relationship between pressure, volume, and temperature.
Gas Laws
Gas laws are empirical relationships that describe the behavior of gases under different conditions. These laws provide a quantitative framework for predicting and understanding gas behavior.
Boyle’s Law
Boyle’s law states that the pressure of a gas is inversely proportional to its volume at constant temperature. This relationship can be expressed mathematically as P₁V₁ = P₂V₂.
Charles’s Law
Charles’s law states that the volume of a gas is directly proportional to its absolute temperature at constant pressure. This relationship can be expressed mathematically as V₁/T₁ = V₂/T₂.
Gay-Lussac’s Law, Behavior of gases worksheet answer key
Gay-Lussac’s law states that the pressure of a gas is directly proportional to its absolute temperature at constant volume. This relationship can be expressed mathematically as P₁/T₁ = P₂/T₂.
Ideal Gas Law
The ideal gas law is a combination of Boyle’s law, Charles’s law, and Gay-Lussac’s law. It provides a comprehensive equation that describes the behavior of an ideal gas under varying conditions.
The ideal gas law is expressed mathematically as PV = nRT, where:
- P is the pressure of the gas
- V is the volume of the gas
- n is the number of moles of gas
- R is the ideal gas constant
- T is the absolute temperature of the gas
The ideal gas law is a powerful tool for predicting and understanding the behavior of gases in various applications.
Real Gases: Behavior Of Gases Worksheet Answer Key
Real gases deviate from ideal behavior under certain conditions, such as high pressure and low temperature. These deviations can be attributed to the attractive forces between gas molecules and the finite volume of molecules.
The van der Waals equation is a modified version of the ideal gas law that takes into account these deviations. The van der Waals equation includes two correction factors: one for intermolecular forces and one for molecular volume.
The van der Waals equation is more accurate than the ideal gas law in predicting the behavior of real gases under non-ideal conditions.
Detailed FAQs
What is the significance of the kinetic molecular theory in understanding gas behavior?
The kinetic molecular theory provides a conceptual framework that explains gas behavior based on the motion and interactions of its constituent particles. It elucidates the relationship between temperature, pressure, volume, and the behavior of gases, enabling us to predict and analyze gas properties under varying conditions.
How does Boyle’s law describe the behavior of gases?
Boyle’s law establishes an inverse relationship between the pressure and volume of a gas at constant temperature. As pressure increases, volume decreases, and vice versa. This principle finds applications in various fields, including scuba diving and the design of gas containers.
What is the ideal gas law, and how does it combine the principles of Boyle’s law, Charles’s law, and Gay-Lussac’s law?
The ideal gas law is a comprehensive equation that combines the principles of Boyle’s law, Charles’s law, and Gay-Lussac’s law. It relates pressure, volume, temperature, and the number of moles of a gas, providing a unified framework for predicting gas behavior under varying conditions.
The ideal gas law is widely used in chemistry, physics, and engineering applications.