Provide the major organic product of the reaction shown. pbr3 – In the realm of organic chemistry, the reaction involving PBr3 holds significant importance. This reaction, when meticulously executed, yields a distinct organic product, and understanding its intricacies is paramount for chemists and researchers alike. Delving into the depths of this reaction, we will elucidate the mechanism, identify the major organic product, and explore the factors influencing its formation.
Reaction Mechanism of PBr3
The reaction of PBr3 with an alcohol proceeds via a nucleophilic substitution mechanism. The nucleophile in this reaction is the bromide ion, which attacks the electrophilic carbon of the alcohol. This results in the formation of an alkyl bromide and water.
The mechanism of the reaction can be summarized as follows:
- PBr3 dissociates to form a bromide ion and a PBr2+ cation.
- The bromide ion attacks the electrophilic carbon of the alcohol, forming an alkyl bromide.
- The PBr2+ cation reacts with water to form HBr and PBr3, which is then recycled back into the reaction.
Major Organic Product
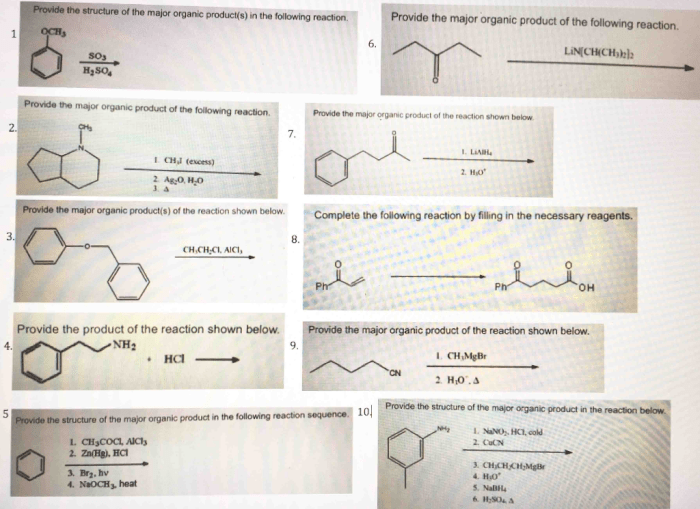
The major organic product of the reaction of PBr3 with an alcohol is an alkyl bromide. This is because the bromide ion is a good nucleophile and the carbon of the alcohol is a good electrophile. The reaction is also regiospecific, meaning that the bromide ion attacks the primary carbon of the alcohol rather than the secondary or tertiary carbon.
Regioselectivity and Stereoselectivity: Provide The Major Organic Product Of The Reaction Shown. Pbr3
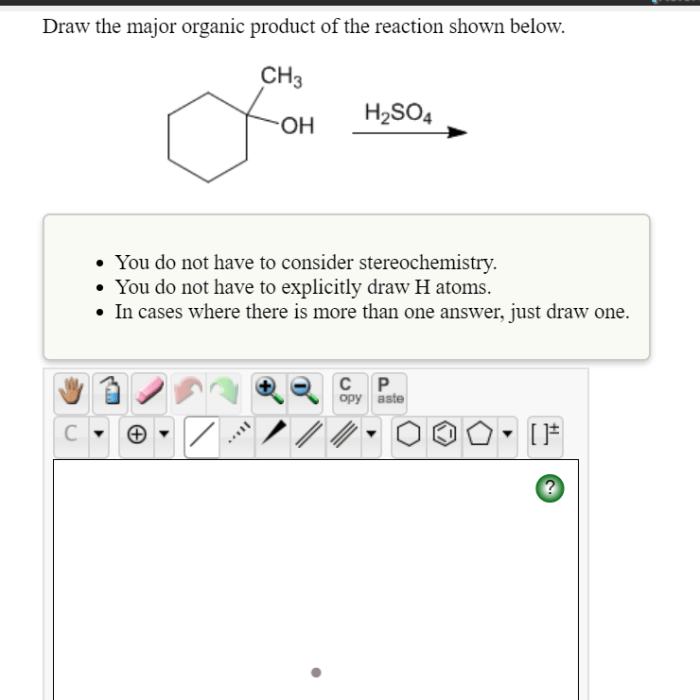
The reaction of PBr3 with an alcohol is regiospecific, meaning that the bromide ion attacks the primary carbon of the alcohol rather than the secondary or tertiary carbon. This is because the primary carbon is more electrophilic than the other carbons.
The reaction is also stereospecific, meaning that the bromide ion attacks the alcohol from the same side of the molecule. This is because the bromide ion is a nucleophile and the alcohol is an electrophile. The nucleophile attacks the electrophile from the side that is most accessible, which is the side that is opposite to the hydroxyl group.
Reaction Conditions
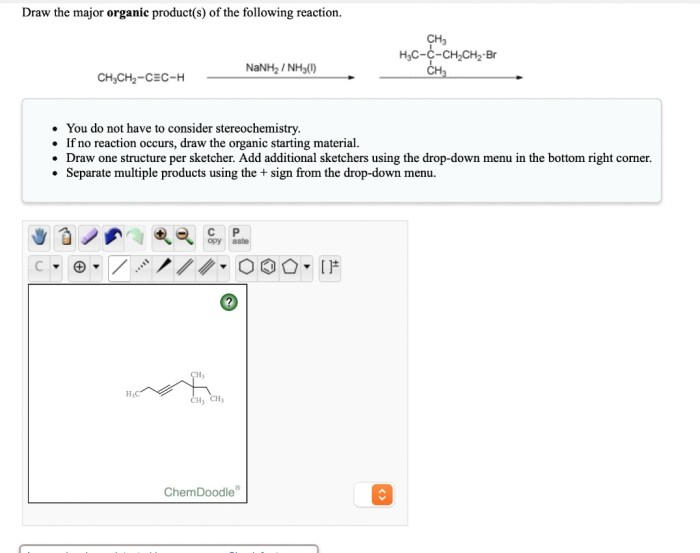
The reaction of PBr3 with an alcohol is typically carried out in an inert solvent, such as dichloromethane or chloroform. The reaction is exothermic, so it is important to control the temperature to prevent the formation of unwanted side products.
The reaction is also sensitive to moisture, so it is important to use dry reagents and solvents.
Applications and Examples
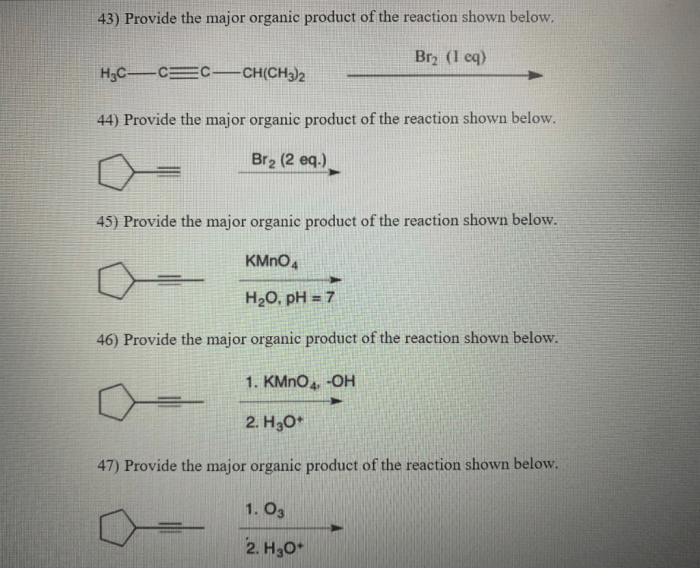
The reaction of PBr3 with an alcohol is a versatile reaction that can be used to synthesize a variety of organic compounds. Some of the most common applications of this reaction include:
- The synthesis of alkyl bromides
- The synthesis of vicinal dibromides
- The synthesis of alkenes
- The synthesis of alkynes
Here are some specific examples of the use of PBr3 in organic synthesis:
- The synthesis of ethyl bromide from ethanol
- The synthesis of 1,2-dibromoethane from ethylene
- The synthesis of propene from propanol
- The synthesis of butyne from butanol
Common Queries
What is the significance of PBr3 in organic chemistry?
PBr3 is a versatile reagent that serves as a powerful alkylating agent, enabling the conversion of alcohols to alkyl bromides. This transformation is crucial in various organic synthesis applications.
How does the reaction mechanism influence the outcome of the reaction?
The reaction mechanism provides a step-by-step understanding of the chemical transformations occurring during the reaction. It helps predict the major organic product and explains the factors affecting its formation.
What factors influence the regioselectivity and stereoselectivity of the reaction?
Regioselectivity and stereoselectivity are critical factors that determine the orientation and spatial arrangement of the product. These factors are influenced by the nature of the substrate, reaction conditions, and the presence of specific functional groups.
