Embark on a scientific odyssey as we delve into the captivating realm of atomic quantification. Our quest today revolves around the enigmatic question: how many atoms are in 0.340 moles of sodium? This inquiry will illuminate the profound connection between macroscopic and microscopic scales, unraveling the mysteries that govern the composition of matter.
Our journey begins with an exploration of Avogadro’s Number, a cornerstone of chemistry that establishes a numerical bridge between the macroscopic world of moles and the microscopic realm of atoms. We will then delve into the concept of the mole, a fundamental unit of measurement that quantifies the abundance of substances.
Armed with these foundational principles, we will embark on the conversion process, transforming moles into atoms, and ultimately unraveling the atomic composition of 0.340 moles of sodium.
How Many Atoms are in 0.340 Moles of Sodium

In chemistry, we often deal with large quantities of atoms and molecules. To make it easier to handle these large numbers, scientists use a unit called the mole. The mole is defined as the amount of substance that contains exactly 6.02214076 × 10 23elementary entities.
These entities can be atoms, molecules, ions, or electrons.
Avogadro’s Number
The number 6.02214076 × 10 23is known as Avogadro’s number. It is named after the Italian scientist Amedeo Avogadro, who first proposed the concept of the mole in 1811. Avogadro’s number is a fundamental constant in chemistry, and it plays a vital role in converting between the macroscopic and microscopic scales.
Mole Concept, How many atoms are in 0.340 moles of sodium
The mole concept provides a convenient way to relate the mass of a substance to the number of atoms or molecules it contains. One mole of a substance is equal to its molar mass expressed in grams. The molar mass of a substance is the mass of one mole of that substance.
For example, the molar mass of sodium is 22.99 g/mol, which means that one mole of sodium weighs 22.99 grams.
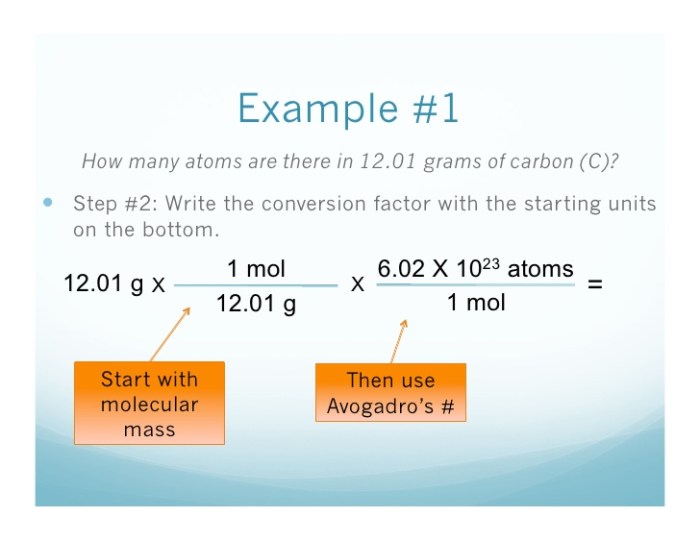
Conversion from Moles to Atoms
To convert from moles to atoms, we can use the following formula:
“`Number of atoms = Number of moles × Avogadro’s number“`
For example, to convert 0.340 moles of sodium to atoms, we can use the following calculation:
“`Number of atoms = 0.340 moles × 6.02214076 × 10 23atoms/mol= 2.05 × 10 23atoms“`
Significance of the Result
The result of this calculation tells us that there are 2.05 × 10 23atoms in 0.340 moles of sodium. This information is useful for a variety of purposes, such as:
- Calculating the mass of a sample of sodium
- Determining the concentration of a sodium solution
- Predicting the chemical reactivity of sodium
FAQ Insights
What is Avogadro’s Number?
Avogadro’s Number is a fundamental constant in chemistry, representing the number of atoms or molecules present in one mole of a substance. It is approximately 6.022 x 10^23.
How do I convert moles to atoms?
To convert moles to atoms, multiply the number of moles by Avogadro’s Number. The formula is: Number of atoms = Number of moles x Avogadro’s Number.
What is the significance of the number of atoms in 0.340 moles of sodium?
The number of atoms in 0.340 moles of sodium provides valuable insights into the composition and properties of sodium at the atomic level. It enables scientists to understand the behavior and reactivity of sodium in various chemical reactions and applications.